Abstract

Background: Inotuzumab ozogamicin (INO) and blinatumomab are superior to chemotherapy for relapsed/refractory B-cell acute lymphoblastic leukemia (ALL). Incorporation of these agents in the frontline setting could improve remission durations and OS in older adults with newly diagnosed B-cell ALL.
Methods: Patients (pts) ≥60 years of age with newly diagnosed Philadelphia chromosome (Ph)-negative pre-B-cell ALL were eligible. Pts were required to have a performance status of ≤3, total bilirubin ≤1.5 mg/dl, AST/ALT ≤3x ULN and creatinine ≤2 mg/dl. Pts received mini-hyper-CVD (cyclophosphamide and dexamethasone at 50% dose reduction, no anthracycline, methotrexate at 75% dose reduction, cytarabine at 0.5 g/m2 x 4 doses) for up to 8 cycles. INO was given at a dose of 1.3-1.8mg/m2 on day 3 of cycle 1 and 0.8-1.3mg/m2 on day 3 of cycles 2-4. Rituximab (if CD20+) and prophylactic IT chemotherapy were given for the first 4 cycles. Responding pts received POMP maintenance for up to 3 years. To decrease the risk of veno-occlusive disease (VOD), the protocol was amended in 3/2017 (pts 50+) to give INO in fractionated doses each cycle (0.6 mg/m2 on day 2 and 0.3 mg/m2 on day 8 of cycle 1; 0.3 mg/m2 on day 2 and 8 of cycles 2-4) and to administer 4 cycles of blinatumomab following 4 cycles of hyper-CVD plus INO, followed by maintenance with 12 cycles of POMP and 4 cycles of blinatumomab (1 cycle of blinatumomab after every 3 cycles of POMP). The cumulative dose of INO given before and after this most recent amendment was 4.3 mg/m2 and 2.7 mg/m2, respectively.
Results: To date, 80 pts have been treated. Six pts were in complete remission (CR) at the time of enrollment. Baseline characteristics are shown in Table 1. The median age was 68 years (range, 60-87 years); 30 pts (39%) were ≥70 years. From all pts, 39% were positive for TP53 mutation and 24% had an adverse-risk karyotype.
Among 74 pts evaluable for morphologic response, 73 (99%) responded (CR, n=66; CRp, n=6; CRi, n=1). MRD negativity by flow cytometry was achieved in 61/76 pts (80%) after 1 cycle and 74/79 pts (94%) overall. The 30-day and 60-day mortality rates were 0% and 3%, respectively.
Among 79 pts who achieved remission, 11 (14%) relapsed, 4 (5%) underwent allogeneic SCT in first remission (1 of whom subsequently relapsed), 32 (40%) are alive in remission without SCT, and 32 (40%) died in remission. Among the 32 deaths in remission, 9 were from sepsis, 3 from VOD, 9 from development of MDS or AML; the rest from miscellaneous causes. Overall, 9 pts (11%) developed MDS/AML after a median of 33 months (range 3-66); 7 of these pts had a detectable TP53 mutation in the myeloid malignancy. Notably, 6 pts (8%) developed VOD, 1 after subsequent allogeneic SCT.
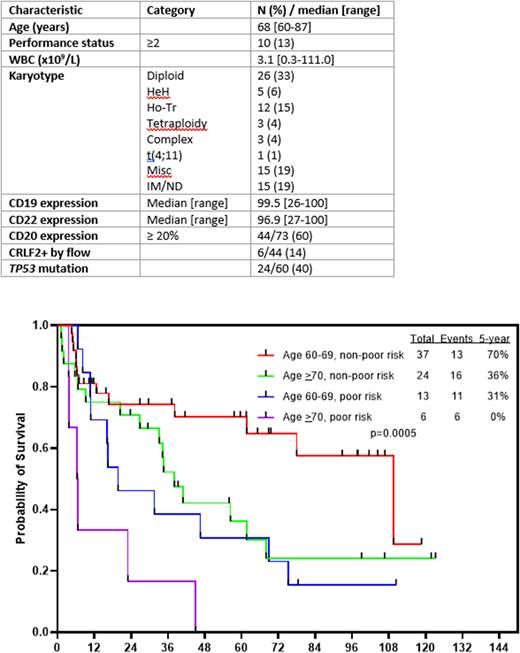
With a median follow-up of 61 months (range, 7-123 months), the 5-year CR and OS rates were 76% and 46%, respectively. Outcomes were superior in pts who were 60-69 years old versus those who were ≥70 years (5-year OS rates: 57% and 28%, respectively; P=0.05) and for those without poor-risk cytogenetics (e.g. KMT2A-rearranged, low hypodiploidy/near triploidy, complex cytogenetics) versus poor-risk cytogenetics (5-year OS rates: 55% and 21%, respectively; P=0.005). The 5-year OS for pts with and without TP53 mutation were 35% and 47%, respectively (P=0.34). The 5-year OS for pts age 60-64 was 65%, for those age 65-69 was 50%, and for those age ≥70 was 28%. The 3-year OS for patients with and without CRLF2+ ALL were 25% and 60%, respectively (P=0.12). Pts ≥70 years of age were more likely to die in remission (19/30 [63%]) and those who were 60-69 years of age (15/50 [30%]) (P=0.004), which was a major driver of the poor survival in this age group. Outcomes were favorable for pts age 60-69 years with poor risk cytogenetics (5-year OS 70%), intermediate for pts age 60-69 with poor risk cytogenetics (5-year OS 31%) or those age ≥70 with low risk cytogenetics (5-year OS 36%), and were very poor for pts age ≥70 with poor risk cytogenetics (5-year OS 0%) (Figure 1).
Conclusion: Low-intensity chemotherapy with hyper-CVD plus INO, with or without blinatumomab, in older adults with newly diagnosed Ph-negative ALL resulted in very high rates of response (99%), including an MRD-negative response rate of 94%. This led to a 5-year OS rate of 46%. Despite the reduced intensity of this regimen, a substantial proportion of pts >70 years of age died in remission. Ongoing efforts are evaluated for INO and blinatumomab without chemotherapy for these pts.
Disclosures
Jabbour:Genentech: Other: Advisory Role, Research Funding; Pfizer: Other: Advisory Role, Research Funding; Bristol Myers Squibb: Other: Advisory Role, Research Funding; Spectrum: Research Funding; Amgen: Other: Advisory Role, Research Funding; Adaptive Biotechnologies: Other: Advisory Role, Research Funding; AbbVie: Other: Advisory Role, Research Funding; Takeda: Other: Advisory Role, Research Funding. Short:AstraZeneca: Consultancy; Stemline Therapeutics: Research Funding; Astellas: Research Funding; Pfizer: Consultancy; Amgen: Consultancy, Honoraria; Novartis: Consultancy; Takeda Oncology: Consultancy, Research Funding. Ravandi:Astellas: Consultancy, Honoraria, Research Funding; Prelude: Research Funding; AstraZeneca: Consultancy; Amgen: Honoraria, Research Funding; BMS/Celgene: Consultancy, Honoraria, Research Funding; Astex/Taiho: Membership on an entity's Board of Directors or advisory committees, Research Funding; Biomea Fusion, Inc.: Research Funding; Syos: Consultancy, Honoraria, Research Funding; Novartis: Consultancy; Amgen: Honoraria, Research Funding; Xencor: Research Funding; Abbvie: Consultancy, Honoraria, Research Funding. Jain:Newave: Research Funding; AstraZeneca: Consultancy, Honoraria, Other: Travel Support, Research Funding; AbbVie: Consultancy, Honoraria, Other: Travel Support, Research Funding; Adaptive Biotechnologies: Consultancy, Honoraria, Other: Travel Support, Research Funding; Janssen Pharmaceuticals, Inc.: Consultancy, Honoraria, Other: Travel Support; Pharmacyclics, Inc.: Consultancy, Honoraria, Other: Travel Support, Research Funding; Fate Therapeutics: Research Funding; Kite, a Gilead Company: Consultancy, Honoraria, Research Funding; Mingsight: Research Funding; Takeda: Research Funding; Servier Pharmaceuticals LLC: Research Funding; Medisix: Research Funding; Dialectic Therapeutics: Research Funding; Novalgen: Research Funding; Loxo Oncology: Research Funding; TG Therapeutics: Honoraria; MEI Pharma: Honoraria; Ipsen: Honoraria; Cellectis: Honoraria, Research Funding; Beigene: Honoraria; TransThera Sciences: Research Funding; Aprea Therapeutics: Research Funding; Pfizer: Research Funding; Incyte Corporation: Research Funding; Cellectis: Honoraria, Research Funding; BMS: Consultancy, Honoraria, Other: Travel Support, Research Funding; ADC Therapeutics: Research Funding; Precision Biosciences: Consultancy, Honoraria, Other: Travel Support, Research Funding; Genentech, Inc.: Consultancy, Honoraria, Other: Travel Support, Research Funding; CareDx: Honoraria. Kadia:BMS: Consultancy, Research Funding; Abbvie: Consultancy, Research Funding; Novartis: Consultancy; Genentech: Consultancy, Research Funding; Regeneron: Research Funding; Astex: Honoraria; cyclacel: Research Funding; Glycomimetics: Research Funding; Agios: Consultancy; Amgen: Research Funding; Delta-Fly: Research Funding; PinotBio: Consultancy; Genfleet: Research Funding; Astellas: Research Funding; AstraZeneca: Research Funding; Pfizer: Research Funding; Ascentage: Research Funding; cellenkos: Research Funding; Iterion: Research Funding; Servier: Consultancy; JAZZ: Consultancy, Research Funding. Alvarado:Jazz Pharmaceuticals: Research Funding; Daiichi-Sankyo/Lilly: Research Funding; Astex Pharmaceuticals: Research Funding; Sun Pharma: Research Funding; BerGenBio: Research Funding; FibroGen: Research Funding. Burger:Gilead: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses, Speakers Bureau; TG Therapeutics: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses, Speakers Bureau; Pharmacyclics LLC: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses, Research Funding, Speakers Bureau; Novartis: Honoraria, Other: Travel, Accommodations, Expenses; Janssen: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses; BeiGene: Consultancy, Research Funding, Speakers Bureau; AstraZeneca: Research Funding. Daver:Agios, Celgene, SOBI and STAR Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees; Kartos and Jazz Pharmaceuticals: Other: Data monitoring committee member; Karyopham Therapeutics and Newave Pharmaceutical: Research Funding; Astellas, AbbVie, Genentech, Daiichi-Sankyo, Novartis, Jazz, Amgen, Servier, Karyopharm, Trovagene, Trillium, Syndax, Gilead, Pfizer, Bristol Myers Squibb, Kite, Actinium, Arog, Immunogen, Arcellx, and Shattuck: Consultancy, Other: Advisory Role; Astellas, AbbVie, Genentech, Daiichi-Sankyo, Gilead, Immunogen, Pfizer, Bristol Myers Squibb, Trovagene, Servier, Novimmune, Incyte, Hanmi, Fate, Amgen, Kite, Novartis, Astex, KAHR, Shattuck, Sobi, Glycomimetics, Trillium: Research Funding. Borthakur:Catamaran Bio, Abbvie, PPD Development, Protagonist Therapeutics, Janssen: Consultancy; Pacylex, Novartis, Cytomx, Bio Ascend: Membership on an entity's Board of Directors or advisory committees; Astex Pharmaceuticals, Ryvu, PTC Therapeutics: Research Funding. DiNardo:Forma: Research Funding; Kura: Honoraria, Membership on an entity's Board of Directors or advisory committees; Foghorn: Honoraria, Research Funding; Astellas: Honoraria; Bluebird Bio: Honoraria; GenMab: Membership on an entity's Board of Directors or advisory committees; AbbVie: Consultancy, Research Funding; LOXO: Research Funding; Novartis: Honoraria; ImmuneOnc: Honoraria, Research Funding; Astex: Research Funding; Notable Labs: Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees; Servier: Consultancy, Honoraria, Research Funding; Bristol Myers Squibb: Honoraria, Research Funding; Gilead: Honoraria; Cleave: Research Funding; Takeda: Honoraria; GlaxoSmithKline: Membership on an entity's Board of Directors or advisory committees; Jazz: Honoraria. Konopleva:AbbVie, Genentech, F. Hoffman La-Roche, Stemline Therapeutics, Amgen, Forty-Seven, Kisoji; Janssen: Consultancy; Stocks, Reata Pharmaceuticals: Current equity holder in publicly-traded company; AbbVie, Genentech, F. Hoffman La-Roche, Eli Lilly, Cellectis, Calithera, Ablynx, Stemline Therapeutics, Agios, Ascentage, Astra Zeneca; Rafael Pharmaceutical; Sanofi, Forty-Seven: Research Funding; Forty-Seven; F. Hoffman LaRoche: Honoraria; Stemline Therapeutics, F. Hoffman La-Roche; Janssen: Membership on an entity's Board of Directors or advisory committees; Reata Pharmaceuticals, Novartis and Eli Lilly: Patents & Royalties. Garcia-Manero:BMS: Consultancy, Honoraria, Research Funding; AbbVie: Honoraria, Research Funding; Novartis: Honoraria, Research Funding; Astex: Consultancy, Honoraria, Research Funding; Curis: Honoraria, Research Funding; Aprea: Honoraria; Gilead Sciences: Research Funding; Acceleron Pharma: Consultancy; Genentech: Honoraria, Research Funding. Wierda:Genzyme: Consultancy; Gilead Sciences: Research Funding; Bristol Meyers Squibb (Juno and Celgene): Research Funding; Juno: Research Funding; Karyopharm: Research Funding; Pharmacyclics LLC: Research Funding; Kite, a Gilead Company: Research Funding; Oncternal Therapeutics, Inc.: Research Funding; AstraZeneca/Acerta Pharma. Inc.: Research Funding; Sanofi: Consultancy; GSK/Novartis: Research Funding; Cyclacel: Research Funding; Xencor: Research Funding; Sunesis: Research Funding; Genentech: Research Funding; Janssen: Research Funding; Loxo Oncology, Inc./Lilly: Research Funding; Miragen: Research Funding; AbbVie: Research Funding. Kantarjian:NOVA Research: Honoraria; Novartis: Honoraria, Research Funding; KAHR Medical Ltd: Honoraria, Membership on an entity's Board of Directors or advisory committees; Pfizer: Honoraria, Research Funding; Daiichi-Sankyo: Consultancy, Research Funding; Astellas Health: Honoraria, Membership on an entity's Board of Directors or advisory committees; ImmunoGen: Research Funding; Ipsen Pharmaceuticals: Honoraria, Membership on an entity's Board of Directors or advisory committees; Jazz Pharmaceuticals: Research Funding; AbbVie: Honoraria, Research Funding; Ascentage: Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Honoraria, Research Funding; Takeda: Honoraria.
OffLabel Disclosure:
inotuzumab and blinatumomab as frontline therapy in Ph- negative-B-cell ALL
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal